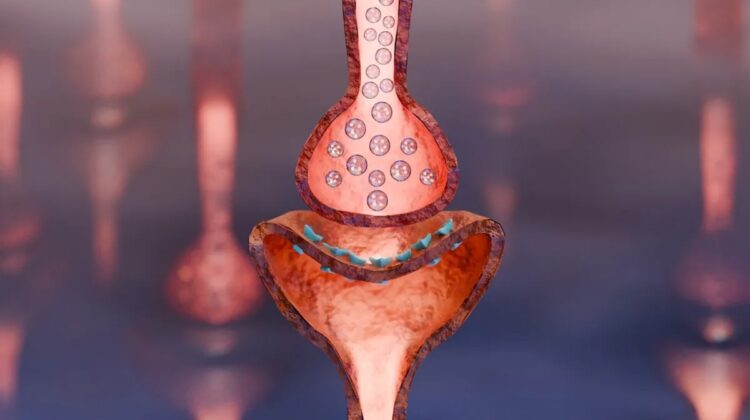
A groundbreaking development in the field of neurology could offer hope to millions of Alzheimer’s patients worldwide. A newly approved drug, Cobenfy, initially designed to treat schizophrenia, is now being explored for its potential to alleviate the debilitating symptoms of Alzheimer’s-related psychosis.
The Science Behind Cobenfy
Cobenfy, a combination of Xanomeline and Trospium, targets specific receptors in the brain. Xanomeline, a muscarinic receptor agonist, has shown promise in improving cognitive function and reducing psychotic symptoms in Alzheimer’s patients. However, its side effects, such as nausea and vomiting, limited its earlier use.
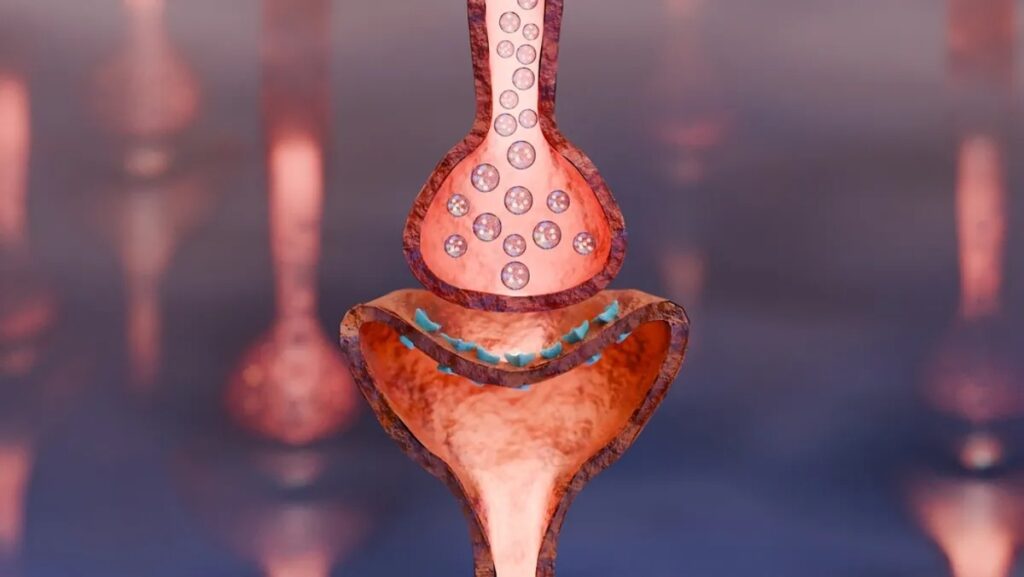
By combining Xanomeline with Trospium, a muscarinic receptor antagonist, researchers have managed to mitigate these adverse effects while preserving the therapeutic benefits. This innovative approach could revolutionize the treatment of Alzheimer’s disease, particularly for patients experiencing psychosis.
Clinical Trials and Future Implications
Pharmaceutical companies are actively conducting clinical trials to evaluate the efficacy and safety of Cobenfy in treating Alzheimer’s-related psychosis. If successful, this drug could significantly improve the quality of life for millions of individuals suffering from this devastating condition.
The potential of Cobenfy extends beyond Alzheimer’s disease. Its mechanism of action suggests that it may also be effective in treating other neurodegenerative disorders characterized by cognitive decline and psychosis.
The Road Ahead
While the future of Cobenfy remains uncertain, it represents a significant step forward in the search for effective treatments for Alzheimer’s disease. As research continues to advance, we can hope for a brighter future for those affected by this debilitating condition.

Leave a Reply