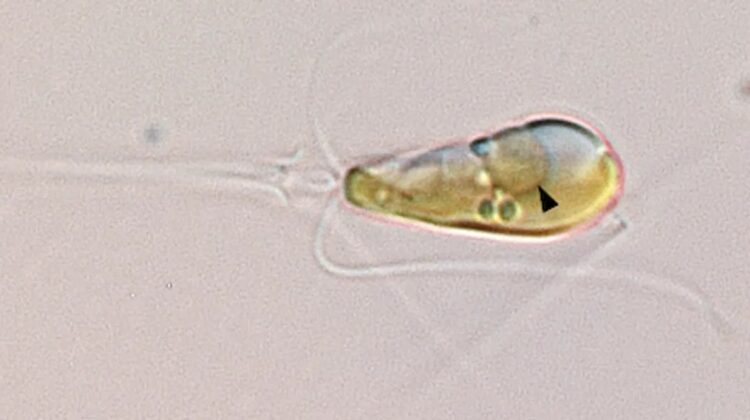
The first-ever nitrogen-fixing organelle in a eukaryotic cell has been confirmed.
In the annals of Earth’s biological history, there are events so rare, so monumental, they occur only once in an eon. And now, amidst the vast expanse of time, one of these extraordinary occurrences has repeated itself. The emergence of a nitrogen-fixing organelle within a eukaryotic cell has been confirmed, marking a momentous milestone in the saga of life on our planet.
This event, known to have unfolded merely thrice before in the chronicles of Earth’s existence, has been documented anew. A marine bacterium, engulfed by its algal host organism, embarked on a journey of co-evolution, eventually integrating itself as an organelle, a vital component of the alga’s cellular machinery. These algae thus become the inaugural eukaryotes, harboring an organelle proficient in nitrogen fixation.
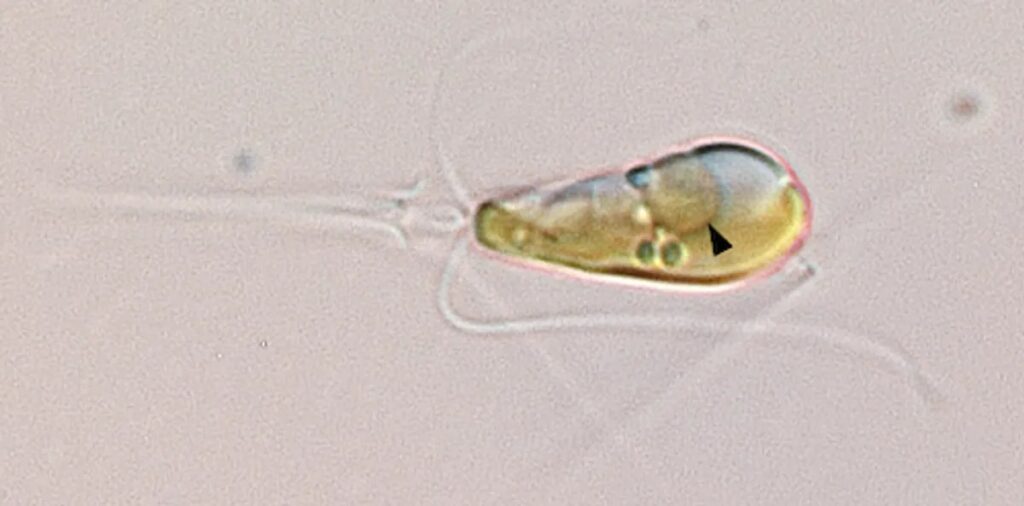
“It’s very rare that organelles arise from these types of things,” remarked Tyler Coale, the lead author of one of the recent papers elucidating this discovery. Indeed, “very rare” might be deemed an understatement. The genesis of such organelles has historically been exceedingly infrequent. The genesis of mitochondria, which catalyzed the dawn of complex life, marked the initial instance. Subsequently, this phenomenon occurred twice more, with the emergence of chloroplasts over a billion years ago heralding the advent of plant life on Earth.
The groundwork for this groundbreaking revelation was laid nearly three decades ago when a team, spearheaded by UC Santa Cruz Professor Jonathan Zehr, unearthed a novel cyanobacterium in the Pacific Ocean, endowed with the capacity for nitrogen fixation. This process, crucial for sustaining life, involves microbes extracting free nitrogen from the environment and amalgamating it with other elements to form essential nitrogen compounds.
Named UCYN-A, the bacterium found its symbiotic partner in a marine alga meticulously cultivated by paleontologist Kyoko Hagino in Japan. Over the ensuing years, the symbiotic relationship between these organisms became increasingly apparent. Recent advancements, however, have unveiled a startling truth: UCYN-A has transcended mere symbiosis to become an integral part of the algal cell, metamorphosing into an organelle.
In two seminal papers published in March 2024, international teams of researchers delineated their findings. The first paper showcased evidence of size ratios between UCYN-A and its algal hosts, indicative of intertwined metabolisms akin to those observed in organelles like mitochondria and chloroplasts. The subsequent paper presented compelling evidence of UCYN-A importing proteins from its host cells, a hallmark of organelle development.
Through meticulous proteomics analysis, Coale corroborated that many proteins essential for UCYN-A’s functionality are synthesized within the algal host and subsequently imported. This integration, likened by Zehr to a “magical jigsaw puzzle,” offers profound insights into the intricate workings of this newfound organelle, dubbed the “nitroplast.”
Unlike its more ancient counterparts, mitochondria and chloroplasts, the evolution of the nitroplast has been dated to approximately 100 million years ago. Already, this discovery has illuminated the impacts of nitrogen fixation on ocean ecosystems and holds promising implications for terrestrial agriculture.
“This system is a new perspective on nitrogen fixation, and it might provide clues into how such an organelle could be engineered into crop plants,” elucidated Coale, hinting at the potential agricultural applications of this groundbreaking research.
While UCYN-A may be the first of its kind to be unearthed, Zehr speculates that others may exist, awaiting discovery. Undoubtedly, this discovery will captivate researchers for decades to come, unraveling the mysteries of nitrogen fixation and reshaping our understanding of cellular evolution.

Leave a Reply