
The concept of extinction has long been synonymous with permanence—until now. Colossal Biosciences, a pioneering genetic engineering company, has made a significant breakthrough in its ambitious mission to revive the woolly mammoth by 2028. In a stunning display of biotechnology, the company has successfully created “woolly mice”—genetically modified rodents that exhibit key traits of the long-extinct mammoth.
A Milestone in De-Extinction: The Birth of the Woolly Mouse
For the first time in 4,000 years, mammoth DNA is once again active in a living organism. Scientists at Dallas-based Colossal Laboratories and Biosciences have engineered mice with two defining characteristics of the ancient mammoth: thick, wavy woolly hair and an accelerated fat metabolism designed for extreme cold survival. These traits were introduced through sophisticated CRISPR gene-editing technology, bringing the world one step closer to witnessing the return of the legendary Ice Age giant.
Colossal’s CEO, Ben Lamm, heralded this achievement as a “watershed moment” in de-extinction science. “By engineering multiple cold-tolerant traits from mammoth evolutionary pathways into a living model species, we’ve proven our ability to recreate complex genetic combinations that took nature millions of years to develop,” he said.
The Science Behind Mammoth Resurrection
Colossal Biosciences has been at the forefront of the de-extinction movement since its founding in 2021. Unlike dinosaurs, which vanished millions of years ago, woolly mammoths roamed the Arctic as recently as 4,000 years ago, and their remains—preserved in permafrost—offer scientists a unique genetic treasure trove. The company has sequenced the genomes of nearly 60 mammoths, using this data to identify the key genes responsible for their adaptation to cold climates.
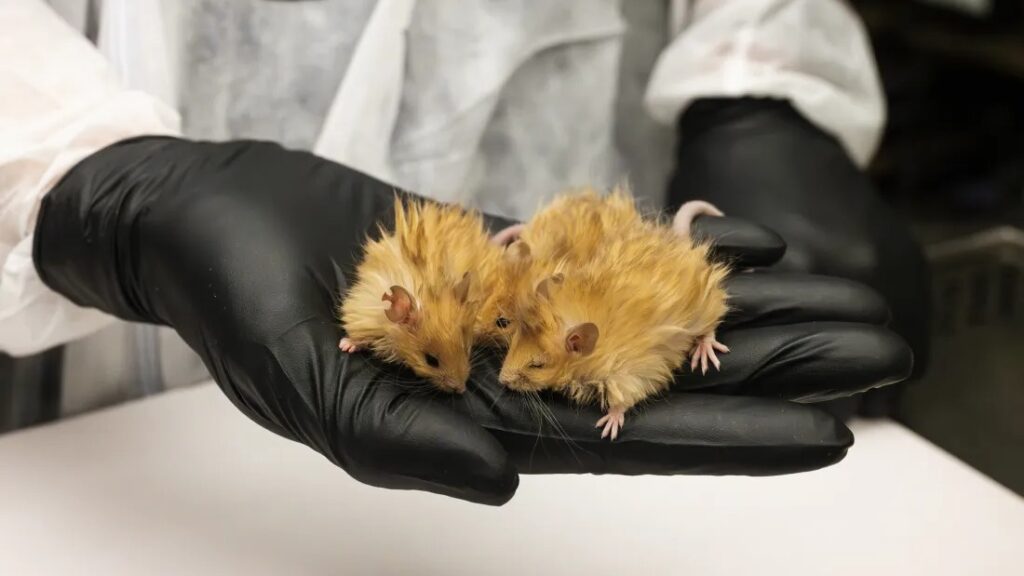
The next step in the process involves transferring these mammoth traits into their closest living relative, the Asian elephant. Scientists plan to edit elephant stem cells to express mammoth-like traits and implant the resulting embryo into a surrogate elephant mother. However, given the elephant’s 22-month gestation period and its endangered status, the researchers first needed a faster and more ethical way to test their gene-editing techniques—leading them to the mouse model.
How Woolly Mice Pave the Way for Woolly Mammoths
The woolly mouse experiment provides a crucial testing ground for genetic modifications before attempting them in elephants. Mice, with their short 20-day gestation cycle and well-understood genome, allow scientists to iterate quickly on their genetic edits.
For this study, Colossal researchers identified seven genes responsible for the mammoth’s shaggy coat, one gene controlling melanin production (which affects coat color), and another regulating lipid metabolism. Using CRISPR, they edited these genes into mouse embryos.
The process wasn’t without challenges. Of the 250 embryos created, fewer than half developed into viable 200- to 300-cell embryos, which were then implanted into surrogate mice. In the end, 38 genetically modified mouse pups were born—each with golden woolly hair and mammoth-like fat metabolism.
While these woolly mice are not directly related to elephants, their successful creation validates Colossal’s gene-editing pipeline and brings the company closer to its ultimate goal. “The woolly mouse project doesn’t bring us any closer to a mammoth, but it does validate the work we are doing on the path to a mammoth,” Lamm explained.

The Road to a Living Mammoth
Recreating a true woolly mammoth involves far more than thick fur and an increased metabolism. Scientists must also engineer genes that regulate the animal’s circulatory system, fat distribution, and cold-resistant adaptations.
Colossal initially targeted 65 genetic edits but has since expanded that number to 85, with the possibility of further refinements. Once the necessary genetic blueprint is perfected, the final step will be creating a viable elephant-mammoth hybrid embryo and successfully bringing it to term.
Beyond Mammoths: The Future of De-Extinction
Colossal Biosciences is not stopping at the woolly mammoth. The company has also set its sights on reviving the dodo and the thylacine (Tasmanian tiger), with the goal of using de-extinction technology to restore lost biodiversity and combat climate change.
Beth Shapiro, Colossal’s chief science officer, emphasized that genetic engineering is not meant to replace traditional conservation efforts but rather to enhance them. “We do not argue that gene editing should be used instead of traditional conservation, but that this is a ‘both and’ situation,” she said. “We should be increasing the tools at our disposal to help species survive.”

Why De-Extinction Matters
With studies predicting that up to 50% of Earth’s species could be extinct by 2050 due to climate change, habitat destruction, and human activity, the need for innovative conservation solutions is more pressing than ever. While reversing extinction won’t undo environmental damage, it could play a role in restoring ecosystems and preventing further biodiversity loss.
By leading the charge in de-extinction, Colossal Biosciences is not only bringing back lost species—it’s also pioneering genetic technologies that could help endangered animals adapt to a rapidly changing planet. If all goes according to plan, the world could see the return of the woolly mammoth within the next four years, marking a historic fusion of science and nature.
A Giant Leap Toward the Ice Age
Colossal’s woolly mouse experiment is a testament to how far genetic engineering has come. While we are still several steps away from witnessing a living, breathing mammoth, the successful manipulation of mammoth DNA in mice proves that the science of de-extinction is no longer just a theoretical dream—it’s becoming a reality.
With continued advancements in biotechnology and gene editing, the return of the woolly mammoth may be closer than we ever imagined. Whether this bold experiment will revolutionize conservation or open a Pandora’s box of unintended consequences remains to be seen. But one thing is certain: the age of de-extinction has officially begun.

Leave a Reply