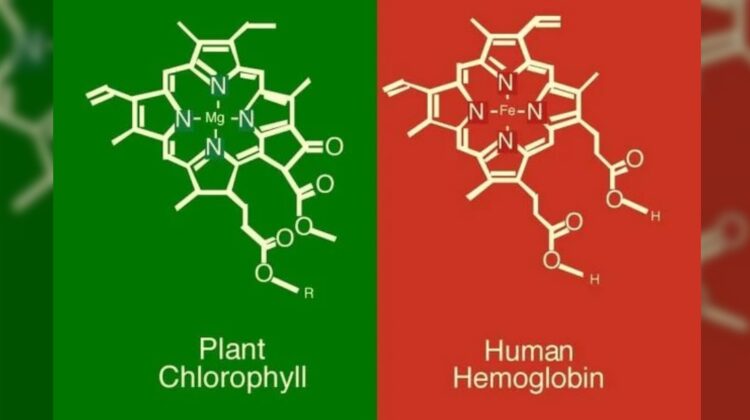
While the terms “chlorophyll” and “hemoglobin” might not seem directly related, these fascinating molecules share a surprising structural similarity. They both play critical roles in sustaining life, but in very different ways.
The most significant difference between chlorophyll and hemoglobin lies at their core. Plants utilize chlorophyll, the pigment that gives them their green color. Hemoglobin, on the other hand, is the iron-containing protein in red blood cells that transports oxygen throughout our bodies.
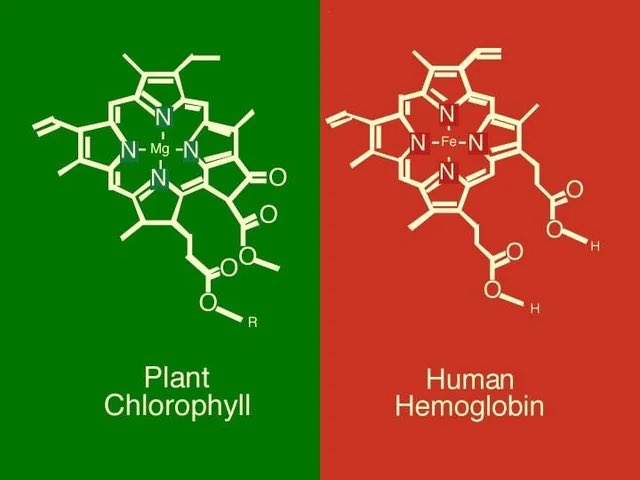
Here’s where the interesting connection comes in: both chlorophyll and hemoglobin have a similar central structure. Imagine a complex ring-like arrangement of atoms. In chlorophyll, a magnesium (Mg) atom resides in the center of this ring. In hemoglobin, an iron (Fe) atom takes its place. This seemingly minor switch in central players has a significant impact on their functions.

Magnesium allows chlorophyll to absorb sunlight effectively, a crucial step in photosynthesis, the process by which plants produce their own food. In contrast, iron within hemoglobin binds to oxygen molecules, enabling their transport from the lungs to tissues throughout the body.
So, while chlorophyll and hemoglobin have a similar architectural base, the difference between magnesium and iron dictates their vastly different functionalities. One harnesses sunlight for life, the other carries the breath of life.

Leave a Reply