
In a remarkable feat of scientific precision, researchers have successfully measured changes within an atom at a mind-boggling scale—the zeptosecond. A zeptosecond corresponds to one trillionth of a billionth of a second, making it the smallest division of time ever observed.
The focus of this groundbreaking study was the captivating event of an electron breaking free from its parent atom when stimulated by light. When photons strike electrons, they become excited and can escape their atomic bonds. This phenomenon, known as the photoelectric effect, was famously described by Albert Einstein in 1905.

Previous investigations into this effect only allowed scientists to observe events after the electron had been ejected from the atom, according to Martin Schultze from the Max Planck Institute of Quantum Optics in Garching, Germany.
Now, Schultze and his team have managed to capture the entire process from start to finish. With zeptosecond precision—equivalent to 10^-21 seconds—they tracked the complete ejection of electrons from a helium atom, achieving a groundbreaking achievement in time measurement.
To conduct their experiments, the researchers employed an ultraviolet laser pulse of astonishing brevity, lasting a mere 100 to 200 attoseconds (10^-18 seconds). By taking numerous readings and analyzing the statistical distribution, they were able to observe events occurring at an incredible rate of 850 zeptoseconds.
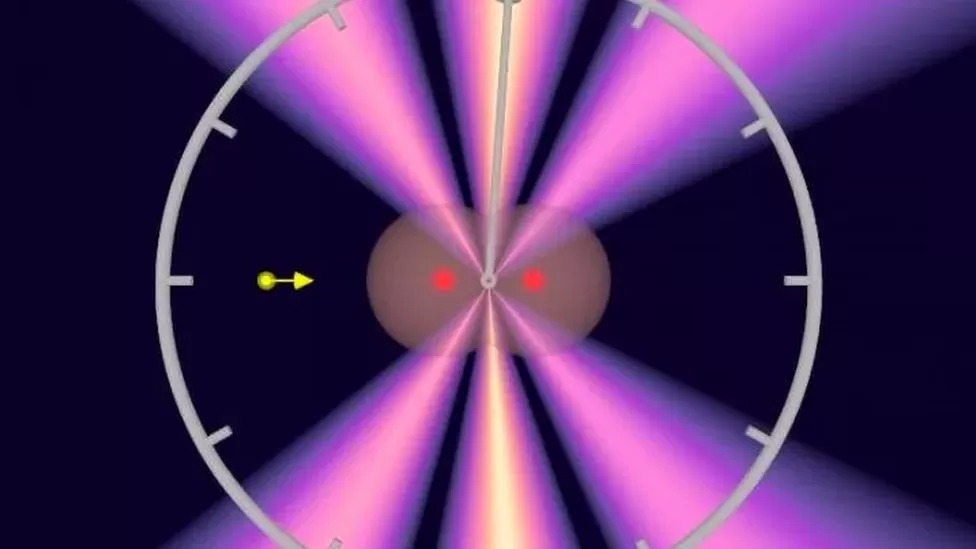
Additionally, a near-infrared laser pulse lasting four femtoseconds (10^-15 seconds) detected the moment an electron escaped from the helium atom. Depending on the electromagnetic field of the laser pulse, the electron either accelerated or decelerated.
Marcus Ossiander, also from the Max Planck Institute, explains, “Using this information, we can measure the time it takes the electron to change its quantum state from the very constricted, bound state around the atom to the free state.”
Schultze notes that the ejections ranged between 7 and 20 attoseconds, depending on the electron’s interaction with the nucleus and the other electron.
The researchers were also able to determine how the electrons shared the laser’s energy, whether evenly or unevenly. In some instances, one electron absorbed all the energy. Several factors influenced this division of energy, including the quantum correlation between the electrons and the electromagnetic state of the laser field.
The choice of helium for the study was deliberate since it possesses only two electrons, enabling the direct measurement of their quantum behavior. For atoms with a larger number of electrons, assumptions would be necessary regarding energy division and ejection time.

These groundbreaking results provide a crucial insight into the quantum behavior of atoms, particularly the workings of electrons. Understanding these dynamics could lead to significant advancements in areas such as superconductivity and quantum computing.
Schultze emphasizes the importance of comprehending electron interactions: “There is always more than one electron. They always interact. They will always feel each other, even at great distances. Many things are rooted in the interactions of individual electrons, but we handle them as a collective thing. If you really want to develop a microscopic understanding of atoms, on the most basic level, you need to understand how electrons deal with each other.”
This extraordinary achievement in measuring time at the zeptosecond scale opens up new frontiers for scientific exploration, enabling researchers to delve deeper into the intricacies of the quantum realm. With each breakthrough, our understanding of the universe expands, pushing the boundaries of human knowledge.

Nice but what do we see in that image ? What is the orange part, the yellow ring, the center red component and the greeish halo ?